In this lab, you'll carry out several simple measurements designed to introduce you to some basic lab skills. Work individually, without a partner. |
Goals
- Learn how to assess the uncertainty in a measurement.
- Learn how to decrease the uncertainty in the measurement (that is, increase the accuracy) of something that repeats in a regular way.
- Use ratio and proportion in an indirect measurement of something that is too large or too small to measure directly with typical laboratory instruments.
Prelab
- You'll need to have completed the Chapter 1 reading and assignments before doing this lab.
- Study this information about precision and accuracy in measurement.
- Assemble the following materials from your lab kit:
- 30-cm plastic ruler
- 3/4-inch diameter steel ball
- white nylon string
- 10 ml graduated cylinder
- eyedropper (in the cylinder)
- tape measure
- Stopwatch or stopwatch application that reads to a precision of at least 0.01 s. See the Software page for possibilities.
Recording your work
You'll record your work in the WebAssign form, L101. While this isn't the only or even the most common way that you'll submit lab work, we're having you use a WebAssign form this time in order that your work can be checked quickly. Therefore, you'll need to have L101 open on WebAssign as you work. Note that the complete instructions for the lab are given below. WA will only point you in the direction of where to enter your responses.There will be 5 numbered items corresponding to the 5 sections below. Within each section, the parts will be labeled with letters the same as below.
1. Uncertainty in measurement
Any measurement has an uncertainty associated with it. A scientist (you) needs to be able to estimate or evaluate the amount of the uncertainty in order to say how good a particular measurement is. As an example, you'll measure the period of a simple pendulum. A simple pendulum is composed of a small, compact object, called a bob, suspended from a string. Set up a pendulum as follows:
- Thread the nylon string through the steel ball and tie a knot in the end to keep the ball from slipping off.
- Suspend the free end of the string from the edge of your desk by taping down the string or stacking something heavy on it. It's important that the string can't slip. The exact length isn't important as long as it remains constant. Something like half a meter is fine.
-
Use the tape measure to measure the distance from the point of support of the string to the center of the ball. Record the measurement in WA L101. Note that due to the difficulty of sighting, a precision of 1 mm is sufficient for this measurement.
-
The period of the pendulum is defined as the time for the bob to swing through one complete cycle. While keeping the string taut, pull the bob to the side so the string is about 10o from the vertical. Release the bob. The time it takes the bob to swing over and then back to you is the period. You'll be timing the period with a stopwatch or stopwatch application that reads to a precision of 0.01 s.
-
Measure the period and record the value in WA L101. Here's a way to improve the accuracy of the method of measurement: Don't start timing as you release the bob. If the bob experiences some friction with your fingers as you release it, that introduces error. Instead, let the bob swing over and back. Start timing as the bob returns to you and comes to a momentary stop. Let the bob continue to swing. Stop timing when the bob returns to you the second time.
-
Take 4 more trials of the period measurement and record them. That will give you a total of 5 trials.
-
Calculate the mean of the 5 time trials.
-
- Examine the list of measurements. Don't be surprised about seeing variability in both the tenths and hundredths digits. That's to be expected. The main source of uncertainty in this measurement is, of course, attempting to synchronize the starting and stopping of the stopwatch with the turnaround point of the bob. These are called endpoint uncertainties. (This is a term that will be used frequently in this course, so we put it in bold. The same is true of all terms in boldface.) One way to assess the amount of uncertainty is simply to estimate it. It would be reasonable to expect an uncertainty of, say, 0.1 s at each endpoint for a total of as much as 0.2 s uncertainty. We say "as much as", because it's possible that one would have endpoint errors that would cancel or partially cancel. So 0.2 s is probably an overestimate. But let's just take that to be the uncertainty for now. The value of 0.2 s is termed an absolute uncertainty, because it's an absolute amount of time. That number is only meaningful in relation to a typical measurement of period. The relative uncertainty is the percentage that the absolute uncertainty is of a representative or average measurement. Let's say a representative measurement is around 1.5 s. The relative uncertainty is then determined as follows.
Relative uncertainty = 100(absolute uncertainty) ÷ (representative measurement) = 100(0.2 s) ÷ (1.5 s) = 10%
Now if you do that calculation, your calculator will return 13.3 repeating. However, the number of significant figures in the result is limited by the number of significant figures in the absolute uncertainty, which has only 1. So we rounded 13.3% to 10%.
When you take several trials of a measurement, there's a mathematical way to determine the uncertainty rather than simply estimating it. You first calculate the deviation of each measurement from the mean of all the measurements. Then you find the mean of the deviations. Here's how to calculate a deviation:
Deviation = |(Value of measurement) - (Value of mean)|.
As you can see, the deviation is defined as an absolute value. If it weren't, then the mean of all the deviations would always be 0. Calculate the deviations and mean deviation and enter the results in WA L101. But read the information in the yellow box below first.
Important note about rounding error
When you submit your result for the percentage mean deviation in WA, you may find that WA checks you wrong even if you're sure you did everything correctly. What may be happening is that you have rounding error from intermediate calculations. When WA does the calculation, it carries many digits and then rounds the final result to the proper number of significant figures. For most purposes, though, it's sufficient to carry an extra digit in intermediate calculations. Here's how this works. What you have to do is jot down the results of intermediate calculations on paper with one digit more than you enter in WA. For example, the mean period in WA would be written with 2 decimal digits, since the individual measurements all have 2 decimal digits. On your paper, though, write the third decimal digit. (If that happens to be a 0, then you don't have to worry about rounding error.) Then, when you jot down the deviations, carry a third decimal digit for them, too. Use that value in calculating the percentage mean deviation. When you actually enter values in WA, though, round them to proper significant figures.
It's a good practice in all your lab work throughout this course to carry at least one extra digit in intermediate calculations in order to minimize rounding error. If you can do the entire calculation in your calculator and round only the final result, that's best.
If you know how to use formulas in Excel, you may find that program to be useful in calculating means and deviations.
- Calculate the relative uncertainty using the mean deviation as the absolute uncertainty and the mean as a typical measurement. In other words, calculate the following:
Relative Uncertainty = Percentage Mean Deviation = 100(Mean Deviation) ÷ (Mean of Measurements).
Before moving to the next part, we should mention that most calculations in experiments require more than one measurement. If, for example, you were calculating speed using measurements of time and distance, each measurement would have an uncertainty. There are methods for combining these individual uncertainties in order to determine the uncertainty in the speed. We'll show you how to do this in the next lab on measuring the speed of sound.
For the next part, keep the pendulum set up as it currently is. Don't change its length.
2. Decreasing the uncertainty in measurement
You may know that the period of a pendulum increases with the length of the pendulum. Suppose you used the same method as previously to measure the length of a longer pendulum. (Don't actually do it now.) Suppose your typical value for the period of the longer pendulum was around 3 s rather than 1.5 s. In that case, the relative uncertainty would be half as much. That's because the endpoint uncertainties remain the same--you're using the same measurement technique--while the denominator in the calculation doubles. This is an example of the following important fact.
|
Now we've told you not to change the length of the pendulum, so you'll need a different way to decrease the relative uncertainty. How can you make the period bigger without changing the length? The answer is to let the pendulum swing through more cycles consecutively between the start and stop of the stopwatch. Let's suppose that you let the pendulum swing through 10 consecutive cycles. You still have the same endpoint uncertainty as before, but the total time measured is 10 times greater. Hence, the relative uncertainty is reduced by a factor of 10. Now you may be thinking this won't work, because the arc through which the pendulum swings gets smaller and smaller with time. However, an interesting fact about the pendulum is that the period is insensitive to the angle of release from the vertical as long as the angle is kept under about 10o. Therefore, the method will work for the pendulum. This is an example of the following important fact.
|
While the point above isn't stated elegantly, it's one of the most important things you can learn in your lab work this year. It applies to measurements of all kinds: time, distance, mass, etc. For labs to follow, we'll generally expect you to decrease the relative uncertainty of your measurements in this way when possible.
-
Now apply the method to measuring the period of the pendulum. After starting the stopwatch, let the pendulum swing through 10 cycles before you stop the watch. As you count cycles, remember that the count at the instant you start the stopwatch is 0. After you've measured the total time for the 10 cycles, enter it in WA.
-
Now calculate the period using the total time. Take 10 to represent an integer; hence, the number of significant figures will the same for the total time as well as the period. This has the effect of adding another decimal digit to the period.
In order to calculate the relative uncertainty for this improved technique, you would, say, take 4 more trials of 10 cycles and then do calculations similar to what you've done before. However, we're not going to require you to do that now, as you'll have the opportunity to use this technique in later labs.
You're finished with the pendulum, so you can take it down now. Be sure to store the materials back in your lab kit where you can find them in the future.
3. Using ratio and proportion in indirect measurements
Your measurement of the length of the pendulum was a direct measurement. That is, you placed the meter stick next to the string and read the endpoints. There are many instances when such a direct measurement cannot be made. This happens when the object measured is too large or too small for the measuring instruments you have available. An example is measuring the height of a tall object such as tree or flagpole. Ratio and proportion comes in handy for such indirect measurements.
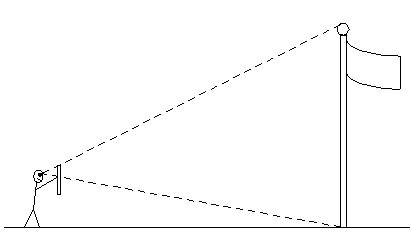 Here's an example. A student uses a meter stick as shown in
the diagram to the the right measure the height of flagpole. She holds the stick
vertically at arm's length and lines up the top of the stick with
the top of the flagpole. Then she moves her thumb down so that it's
in line with the bottom of the pole. In this position, she notes the
position of her thumb on the ruler. While she's holding the stick, a
partner then measures the distance from Crystal's eye to the stick with a ruler. Finallly, the students uses a tape measure the distance to the flagpole along the ground. At this point, the student knows three distances, which we give the
following symbols:
Here's an example. A student uses a meter stick as shown in
the diagram to the the right measure the height of flagpole. She holds the stick
vertically at arm's length and lines up the top of the stick with
the top of the flagpole. Then she moves her thumb down so that it's
in line with the bottom of the pole. In this position, she notes the
position of her thumb on the ruler. While she's holding the stick, a
partner then measures the distance from Crystal's eye to the stick with a ruler. Finallly, the students uses a tape measure the distance to the flagpole along the ground. At this point, the student knows three distances, which we give the
following symbols:
drt = distance from top of ruler to thumb
der = perpendicular distance from eye to ruler
def = perpendicular distance from eye to flagpole
Using the theorem from geometry that the ratios of corresponding sides of similar triangles are equal, we see that h / drt = def / der, where h is the height of the flagpole. This being the unknown, we solve for h to yield
h = drt(def / der).
The accuracy of this measurement of h is limited by the two smaller measurements of drt and der. It's not easy to hold a meter stick steady while sighting two positions on it. If the angle of sight is off even by just a degree, that is magnified into a large distance error at the position of the flagpole.
You'll be using the graduated cylinder and eyedropper next. The glass eyedropper is stored inside the cylinder for protection from breakage. Remove the bulb of the eyedropper from the cylinder and then slide the glass tube out. Snap the bulb onto the glass tube if not already assembled.
The exercise that you'll do next will help you get a handle on how uncertainties can be magnified in an indirect measurement. The exercise is to determine the number of drops in a liter of water. Of course, you're not going to fill a liter with drops one at a time. You've probably guessed that you'll used the 10 ml graduated cylinder. You could fill that with drops and then use a ratio and proportion method to extrapolate to a liter. We don't recommend, though, that you take the time to fill the entire cylinder with drops. Use the following procedure instead:
-
First, add some water to a cup from your kitchen. You'll add water to the cylinder from this cup. Pour enough water into the cylinder to bring the level up about halfway. Read the water level. When you do, be sure to either get down to the level of the cylinder. The smallest divisions are 0.2 ml, but you can estimate halfway between to provide a precision of 0.1 ml. Read the water level to 0.1 ml and record it in WA L101.
-
Fill the dropper with water and start adding drops to the cylinder, trying to drop them in the center rather than on the sides. (This is why we had you fill the cylinder up halfway to begin with. It's easier to keep the drops off the side in a shorter fall.) 50 drops will be enough to add. When you're finished, read the water level again. Record that and the number of drops in WA.
- Now calculate the number of drops in a liter. If your answer is counted wrong in WA, your significant figures may be incorrect. Don't worry about it now as this won't be counted against you. You'll have another chance at this in part f.
You're generally not done with a measurement until you've assessed the uncertainties quantitatively or at least taken note of potential errors in a qualitative way. Keep in mind that there's nothing wrong with an error in an experiment. In lab science, the word error isn't synonymous with mistake. Think of a mistake as the result of some carelessness such as a mistake in a calculation. In the flagpole experiment, a mistake would be measuring the perpendicular distance from the meter stick to the flagpole instead of the perpendicular distance from the eye to the flagpole. Such things can easily be avoided with care in calculation and critical thinking about the experimental procedure. An error, on the other hand, is due to an inherent uncertainty in the method. For example, the student might have tilted the meter stick slightly away from the vertical or shifted the meter stick up and down slightly while sighting.
Having said all that, you'll now assess the uncertainty in your indirect measurement of the number of drops in a liter.
-
Calculate the relative uncertainty in the volume reading. For the numerator, you'll use twice the endpoint uncertainty of 0.1 ml, since you were supposed to read to that precision. Use as the denominator the volume of water corresponding to the number of drops you added. This would be the difference between the two readings. As in part c, if your answer is counted incorrect, this may be due to incorrect significant figures. Again, this won't be counted against you, and you'll have another try in part f.
-
Calculate the absolute uncertainty in the number of drops in a liter. This simply involves solving the relative uncertainty formula for the absolute uncertainty.
Relative uncertainty = 100(absolute uncertainty) ÷ (representative measurement)
Absolute uncertainty = (Relative uncertainty/100)(representative measurement)
For the relative uncertainty, use the result of your calculation in step d. For representative measurement, use your calculated number of drops in a liter. This works because the only source of uncertainty in this measurement is assumed to be the endpoint uncertainties. The number of drops you counted is taken to be an integer, and the volume of 1 liter is taken to be a given, having no uncertainty.
-
Let's suppose that you calculated there were 65,293 drops in a liter and that the uncertainty was 1,150 drops. (These numbers are chosen wildly and aren't rounded to appropriate significant figures.) This tells you that the figure of 65,293 is uncertain in the thousandths digit and hence, in all digits to the right of the thousandths place. Therefore, it's nonsensical to say you determined 65,293 drops, because you really have no idea what the last three digits are. Based on your uncertainty estimate, you can say that the 5 isn't complete uncertain; it might be a 4 or a 6. With this in mind, the appropriate way to express the measurement, being mindful of the uncertainty, is 6.5 x 104 ± 1 x 103 drops. Note that we used powers of ten notation, since that's the best way to indicate the significant digits when 0s are involved. If we wrote 65,000 ± 1,000 drops, it wouldn't be clear if the 0s were intended to represent significant digits. See the guide on signficant figures if you need to review this. Now write your result for the number of drops in a liter ± absolute uncertainty, rounded appropriately and expressed in powers of ten notation. In WA, 65,000 is written 6.5E4.
- Your calculation of the absolute uncertainty in the drops in a liter measurement was quantitative, because it involved numbers. However, there are also errors that are qualitative. We can identify and describe them in words but don't have an easy way to quantify them. When you describe a potential source of error, you should say how you would expect it to affect the results and why. Would you expect the error to yield a result that's smaller (or larger) than the actual value? Such an error is termed systematic. Or would the error be random in the sense that it might yield smaller results in some trials and larger in others. Here's an example relating to the period of a pendulum measurement. The following would be an acceptable description of a potential source of error.
Suppose the length of the pendulum were actually longer than what you measured. In that case, all of your period measurements would be greater than one would expect for the length that you measured, since the period of a pendulum increases with length. This would be a systematic error.
Now describe in words a potential source of error in the drops measurement, an error that hasn't been previously mentioned. With that stipulation, you wouldn't discuss the uncertainty in reading the water level, because you've already quantified that. Include the following in your response: i) description of what the error is, ii) what type of error it is, random or systematic, iii) what effect it has on the result (larger, smaller?) and why.
You're finished with the stopper and graduated cylinder. After the cylinder and dropper have dried, repackage them for safe storage the way you found them.
4. Applying what you've learned
Now you'll apply the techniques that you've learned above to do the following: Using your centimeter ruler only, measure the thickness of a sheet of paper (2 pages) in your textbook to within an absolute uncertainty of 1 micron. A micron is 1 x 10-6 m or 1 x 10-3 mm. That's one-thousandth of the smallest division on your ruler.
Of course, this will need to be an indirect measurement. You'll obviously need to measure more than one sheet of paper at a time. How many sheets will you measure in order to get the smallest possible uncertainty, given that you can only use a centimeter ruler? In order to succeed in the goal, you'll need to do the following:
- Select a number of sheets N that will minimize the uncertainty.
- Measure the thickness of the N sheets with your centimeter rule to a precision of 0.1 mm. That's a tenth of the smallest division.
- Develop a technique of holding the pages in order to measure as accurately as you can.
- Determine the absolute uncertainty in your result for the thickness of a sheet of paper. (See the previous exercise as a guide.)
Textboxes are provided in WebAssign for you to do the following:
- In a few sentences, describe your measurement method in words with the emphasis on telling what steps you took to make the most accurate measurement you could.
- List your data, labeling it clearly in words. Then show your calculation of the thickness of a sheet of paper. Round to appropriate significant figures.
- Show how you determine the absolute uncertainty in your determination of the thickness of a sheet. Make this descriptive as well quantitative. use words to describe what you're doing, and show your calculations. Round appropriately.
- Convert the uncertainty calculated in part c to microns to determine whether you met the goal.
5. Conclusion
Nearly every lab you do will have a conclusion. Typically, this is a summary of what you did, what you found out, and whether you achieved the goals of the experiment. Since the goals of the present experiment are to learn particular techniques, in your conclusion describe in a well-composed paragraph what you learned about accuracy and uncertainty in measurement.